Battery Maintenance
Author: Richard Kenney
Batteries are a key element in Greenpower racing. Because they are inert boxes which don't make noise, get hot or move around (unless something is seriously wrong - which CAN happen!) it is easy to take them for granted. This is of course a mistake. The lead acid batteries that we use have a lifetime and wear out after a lot of use. They are also quickly damaged if they are not looked after properly.

How do batteries work?
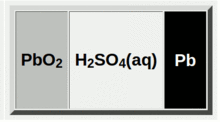
The active ingredients in a lead acid battery are just lead and acid - sulphuric acid. Actually that's not quite true, the positive plates are lead oxide. So the basic battery is a plate of lead oxide and a plate of pure lead, separated by some sulphuric acid as shown on the right.
This is a cell, and in the charged state the voltage difference between the lead oxide plate and the lead plate is 2 volts. The batteries we use have six cells inside which are connected in series to make a 12 volt battery.
When the battery is used a current passes from one terminal through the outside circuit and back to the other terminal. This is another way of saying that electrons flow out of the lead plate through the outside circuit and back to the lead oxide plate. Where do these electrons go inside the battery?

Each time an electron comes out of the lead plate some of the lead reacts with the sulphuric acid to create lead sulphate and water. In the same way at the lead oxide plate when an electron comes in some lead oxide reacts with the sulphuric acid to create lead sulphate and water. As the battery discharges more and more of the plates and acid are converted to lead sulphate and water until the plates are both completely lead sulphate and the battery is completely flat.
By forcing current 'backwards' through the battery the process is reversed and the battery is recharged.
So why do batteries need looking after and why do they wear out?
Each time a battery is discharged lead sulphate is formed on the plates. Lead sulphate can exist in several forms and while initially it is created as an amorphous form which can be dissolved, some will crystallise. This crystallised lead sulphate cannot be converted back and plays no useful purpose. In fact it protects the material underneath from the acid and so reduces the active surface area of the plate. The longer the battery is left not fully charged the more crystallised lead sulphate is formed and eventually there is no active surface left to make the battery work - this is known as sulphation and is why it is important to fully charge used batteries as soon as possible.
Another failure mechanism is overcharging. Greenpower batteries have the electrolyte absorbed in glass fibre matting between the plates. This is why it is OK to tip the batteries up and why they are safe to transport - there is no free liquid inside. This does cause one problem though. If the battery is overcharged then some of the electrolyte is converted to hydrogen and oxygen gasses. This is why batteries should be charged in a ventilated area (hydrogen and oxygen is an explosive mixture so you don't want a concentration building up!). The batteries are designed to release the gas safely but there is no way to replace the lost electrolyte. If the battery is overcharged consistently then the capacity will reduce as the amount of electrolyte reduces. A secondary issue is corrosion of the positive plate caused by the evolved gasses.
Checking Battery Health
To check the health of our batteries we perform a discharge test. To do this we measure how long it takes a fully charged battery to become fully discharged when a standard load is applied. We then record the results for each battery so that we know which ones to use for important races. We repeat the measurements regularly (but not often!) as the batteries age and it becomes necessary to retire the worst ones and buy new.
To apply the load we have built a discharge tester. It is just a large heatsink with two power resistors attached and a small control circuit to switch off the load as the battery becomes discharged.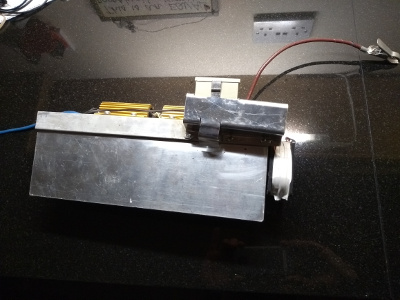
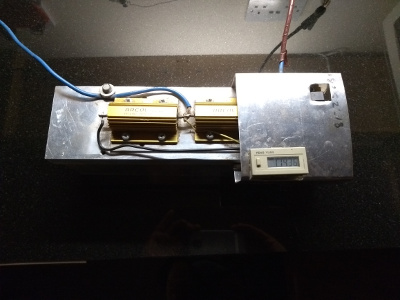
We have used a self contained timer which is controlled by the voltage across the resistors but these seem to be difficult to find at a reasonable price so an alternative for future builds would be an Arduino with an LCD display. This would also make an good introductory project to Arduino programming. Bear in mind though that the Arduino would need a separate power source.
